Skyhawk’s SKY-0515 demonstrated dose-dependent huntingtin (HTT) mRNA reduction in healthy volunteers, with 72% reduction at the highest dose tested in the multiple ascending dose study
SKY-0515 was generally well tolerated at all doses tested
Given these positive topline results, we anticipate dosing in the patient arm of the study in Q3 2024 and initiation of a Phase 2 study early next year
BOSTON, July 10, 2024 (GLOBE NEWSWIRE) -- Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies designed to modulate critical RNA targets, today announced positive results from Parts A and B of its Phase 1 clinical trial of SKY-0515, which is being developed as a potential treatment for Huntington’s disease (HD). SKY-0515 demonstrated an average HTT mRNA reduction of 72% at a daily oral dose of 9mg and was generally well tolerated at all doses tested.
SKY-0515 is Skyhawk’s investigational small molecule RNA splicing modifier developed through the company's novel RNA-splicing platform. SKY-0515 is designed to reduce both HTT protein and PMS1 protein, an additional key driver of somatic CAG repeat expansion and HD pathology.
“Huntingtin-lowering and somatic expansion have been two of the hottest targets in HD research in the past decade. Reducing both HTT and PMS1 could have greater therapeutic benefit than lowering HTT alone,” said Ed Wild, Professor of Neurology at University College London. “Huntington’s disease is a rare hereditary neurodegenerative disease affecting over 40,000 patients in the United States. There are no approved treatments that can reverse or slow its course of progression. SKY-0515’s HTT reduction has the highest dynamic range I’ve seen from any therapeutic modality and gives me great hope for SKY-0515’s potential to one day help those patients in need.”
“We believe that, with these impressive HTT mRNA lowering results and the drug’s predicted suppression of the PMS1 protein, SKY-0515, if approved, could make a meaningful difference in Huntington’s patients’ lives,” said Douglas V. Faller, M.D., Ph.D., Chief Medical Officer, Skyhawk Therapeutics. “The Safety Review Committee has determined that SKY-0515 has been generally well tolerated at all tested doses with a dose proportional increase in systemic exposure and, given these favorable safety results, approved this study to move into the patient arm. Recruitment has begun, and topline data from this part of the trial are expected to report in Q2 2025.”
“After initiating this Phase 1 clinical trial in late 2023, we’re delighted with the speed at which we’ve conducted this study and thrilled to report such compelling results for SKY-0515,” said Clint Musil, Chief Executive Officer, Skyhawk Therapeutics. “These topline data represent a crucial step forward for SKY-0515 and demonstrate the immense potential of the Skyhawk platform to target indications for which there are no approved disease modifying therapies.”
HTT mRNA levels in the blood from pre-dose are described in the charts below.
| SAD: Maximum reduction in HTT mRNA level in blood from pre-dose within 24 hours after single dose | MAD: Average reduction in HTT mRNA level in blood from pre-dose over 24 hours post dose on day 14 | |
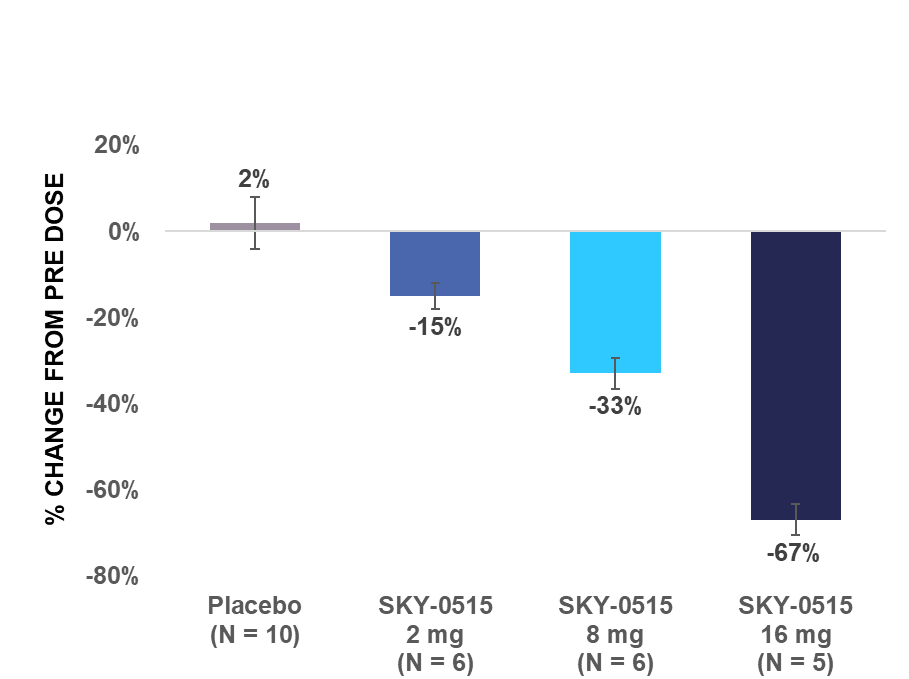 | 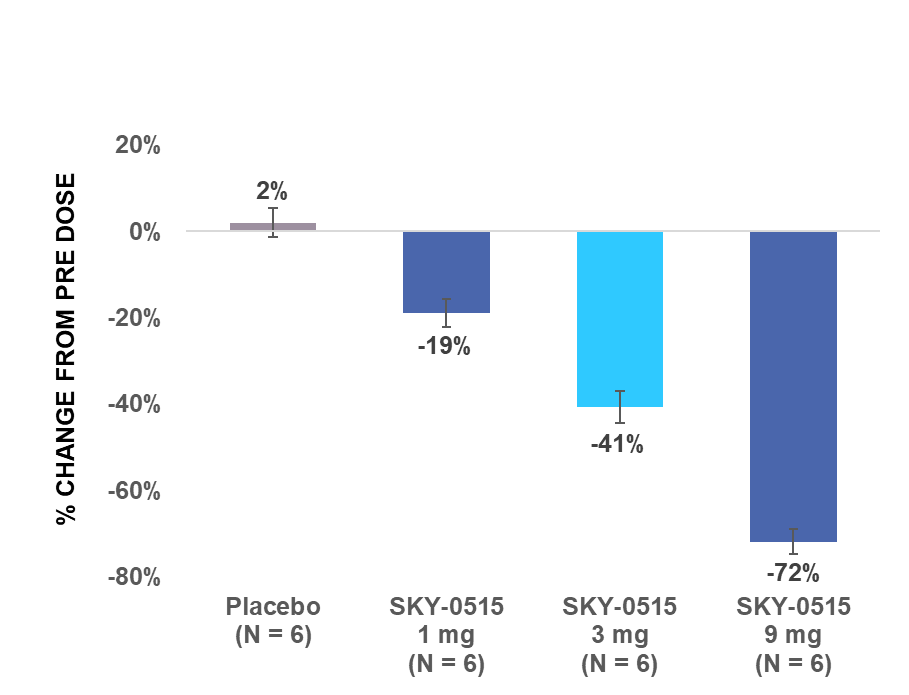 | |
| Note: Error bars represent standard error of the mean. | ||
About SKY-0515 Phase 1 Clinical Study
SKY-0515 is currently being evaluated in a Phase 1 clinical trial. The Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics, specifically blood biomarker modulation activity, of SKY-0515 in healthy volunteers and individuals with early-stage Huntington’s disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in healthy volunteers.
Part A was a double-blind placebo-controlled single ascending dose study in healthy adult volunteers. In Part A, five cohorts of participants were dosed with escalating single doses of SKY-0515 ranging from 1mg to 16mg or placebo. Additionally, the influence of food on the pharmacokinetics of SKY-0515 was examined in a dedicated cohort.
Part B was a double-blind placebo-controlled multiple ascending dose study in healthy adult volunteers. In Part B, three cohorts of participants were randomized to receive multiple ascending doses of SKY-0515 ranging from 1mg to 9mg or placebo administered daily from days 1 to 14 (inclusive). Dose levels of SKY-0515, identified in Parts A and B, will be evaluated in Part C.
Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo of individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) preceded by an observational period lasting a minimum of 28 days, which aims to evaluate pharmacodynamic parameters such as mutant HTT protein and mRNA. Recruitment for Part C has begun, and topline data are expected in Q2 2025.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company focused on the discovery and development of novel small molecule therapies designed to modulate critical RNA targets and revolutionize patient treatment for some of the world’s most intractable diseases. Skyhawk’s discovery expertise is rooted in its proprietary drug discovery platform, which assesses, identifies, and tests RNA splicing targets and small molecules across a broad range of therapeutic areas and disease states. Skyhawk has built collaborations with multiple pharma partners that leverage Skyhawk’s novel platform across disease areas including neurodegenerative disease, autoimmune disease, and oncology. For more information visit www.skyhawktx.com.
Investor Contact
Anne Marie Fields
Precision AQ (formerly known as Stern Investor Relations)
annemarie.fields@precisionaq.com
332-213-1956
Skyhawk Contact
Maura McCarthy
maura@skyhawktx.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4b26e782-86d2-402d-a4e1-d7892ee21fad
https://www.globenewswire.com/NewsRoom/AttachmentNg/23bc1da1-bf4a-42a8-9d28-1b54d09b7413

source: Skyhawk Therapeutics
《說說心理話》心理急救II:幾個徵兆辨認身邊人需要心理支援!點樣對情緒進行急救、自我照顧?專家分享穩定情緒小練習► 即睇
































