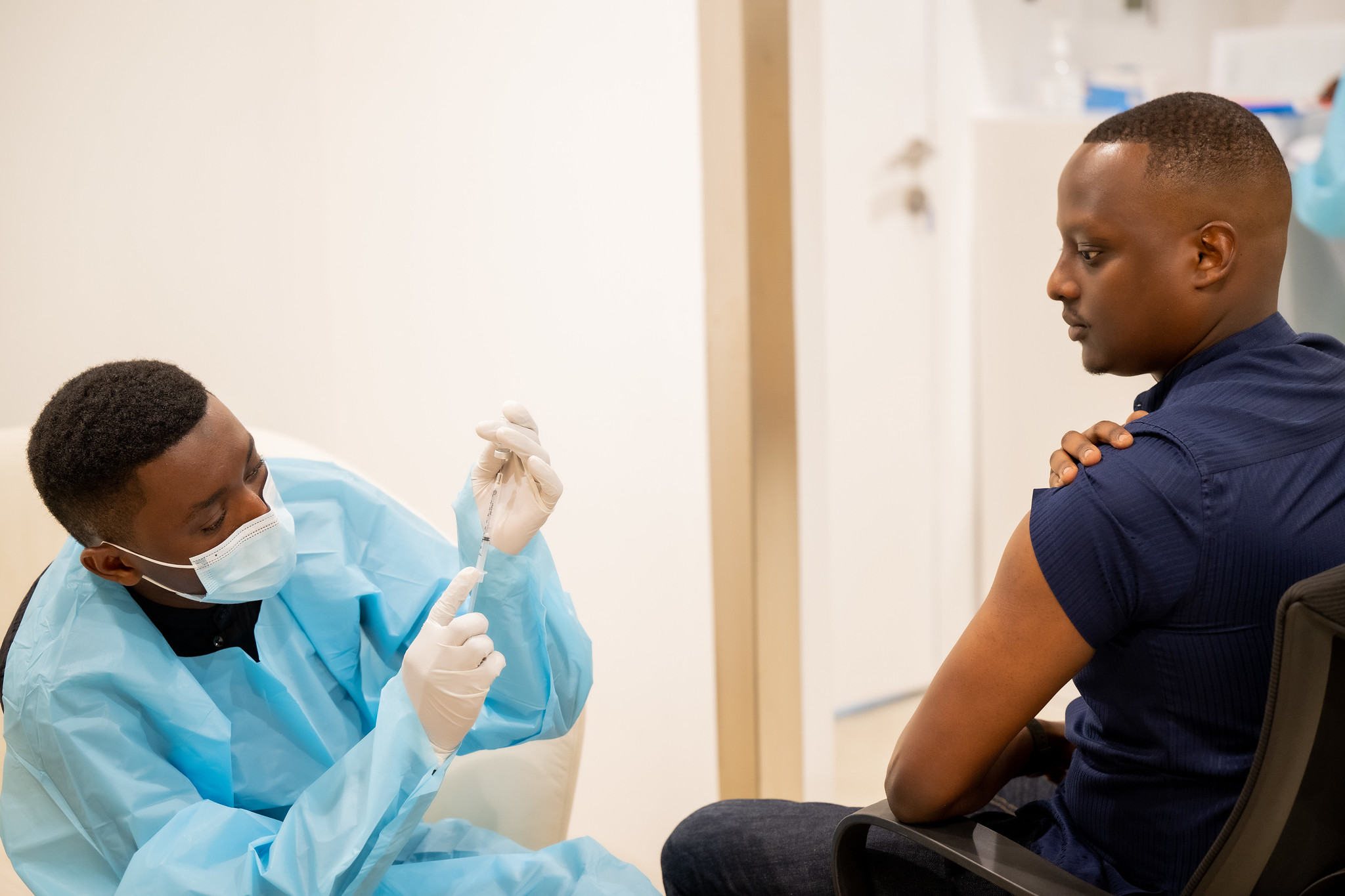
Photo caption: Rwanda Minister of State for Health, Yvan Butera, was among those who received Sabin’s investigational vaccine in the open-label clinical trial.
WASHINGTON, Oct. 31, 2024 (GLOBE NEWSWIRE) -- In continued collaboration with Rwanda to address the Marburg virus outbreak, the Sabin Vaccine Institute has dispatched approximately 1,000 additional investigational vaccine doses for a randomized clinical trial arm within the ongoing open-label study. More than 1,500 frontline workers have already been vaccinated in Rwanda with the Sabin vaccine.
Under the updated protocol, sponsored by the Rwanda Biomedical Center, approximately 1,000 at-risk individuals, including mine workers, will receive Sabin’s single-dose investigational vaccine in a 1-to-1 randomization. Half will receive the vaccine immediately, and the other half 21 days later to align with the end of the disease incubation period.
Genomic sequencing of the index case (first identified case in an infectious disease outbreak) in Rwanda suggests a zoonotic origin, with strains similar to those found in fruit bats in a mine.
The new trial arm will assess safety, immunogenicity, and efficacy. Pending a request from Rwandan officials and authorization from the U.S. Administration for Strategic Response and Preparedness, Sabin plans to supply additional vaccines for this portion of the trial.
“As Rwandan health officials determined, the most expeditious and effective way to reach this new group of people impacted by the outbreak is to adapt the current protocol,” says Sabin CEO Amy Finan. “While this vaccine is still investigational, our mission is to ensure that knowledge can move swiftly from research to real-world solutions, with scientific rigor and safety as our highest priorities.”
At a news conference today about the Marburg outbreak, Rwanda minister of state for health Yvan Butera also addressed the need for more vaccine doses. Since the outbreak began, Sabin has been working directly with Rwandan officials and partners to mount a coordinated response.
As in previous outbreaks, Sabin continues to collaborate closely with its contract manufacturer, ReiThera, to prepare and ship the vialed doses to Rwanda.
Over 1,700 vaccines have already been delivered to Rwanda, with the first shipment of doses arriving just nine days after the outbreak was declared on September 27. The initial part of the trial focused largely on health workers, a group that suffered the most casualties in this outbreak.
Rwanda has confirmed 66 Marburg cases in what is one of the largest recorded outbreaks of the disease. Case numbers declined sharply within two weeks, with 15 deaths reported so far. The case-fatality rate, approximately 23%, remains significantly lower than previous outbreaks, which have seen mortality rates as high as 88%.
Designed to prevent illness before exposure to the virus, Sabin’s Marburg vaccine has not yet been proven to have clinical benefit for recipients of the vaccine. The candidate is currently in Phase 2 trials in Uganda and Kenya with no safety concerns reported to date. In non-human primates, it has demonstrated rapid immunity within one week, and in Phase 1 trials, it has shown safety and immunogenicity in humans.
Marburg virus disease remains a serious threat, with a high case fatality rate, and no approved vaccines currently available. Symptoms typically emerge between two and 21 days after infection.
Since 2019, Sabin has been at the forefront of advancing vaccines for filoviruses based on the cAd3 platform. Interim results from the Marburg trial are expected next year, with a U.S.-based trial planned for 2025. Sabin is also a key partner in MARVAC, a WHO-coordinated effort promoting global collaboration in Marburg vaccine development.
Sabin’s development program, which includes clinical trials and manufacturing of clinical trial material that have been leveraged for this current outbreak, is supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, under multi-year contracts. To date, BARDA has obligated $235 million to Sabin for advancing vaccine research and development against Sudan ebolavirus and Marburg virus diseases.
In addition to BARDA and Rwanda’s government, Sabin is grateful for all the organizations including CEPI, GSK, IQVIA, kENUP Africa, National Institutes of Health’s Vaccine Research Center, PPD, WHO, and World Courier who have contributed to our past and current efforts.
About the Sabin Vaccine Institute
The Sabin Vaccine Institute is a leading advocate for expanding vaccine access and uptake globally, advancing vaccine research and development, and amplifying vaccine knowledge and innovation. Unlocking the potential of vaccines through partnership, Sabin has built a robust ecosystem of funders, innovators, implementers, practitioners, policy makers and public stakeholders to advance its vision of a future free from preventable diseases. As a non-profit with three decades of experience, Sabin is committed to finding solutions that last and extending the full benefits of vaccines to all people, regardless of who they are or where they live. At Sabin, we believe in the power of vaccines to change the world. For more information, visit www.sabin.org and follow us on X, @SabinVaccine.
Media Contact:
Monika Guttman
Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 662-1841
press@sabin.org
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f38d0939-533d-4fea-8d71-882be4461b28

source: Sabin Vaccine Institute
【你點睇?】新一份預算案公布,多項開源節流措施推出,你認為哪一項措施最能達致改善政府財赤目標?► 立即投票






























